Scientists discovery of an X-ray technique that will ‘transform the world’
Scientists have been able to use X-rays to study a singular atom properly for the first time.
If you’ve ever broken a limb, you’ll likely have gone for an X-ray and gone through the process of passing through some electromagnetic radiation to then see an image of your bodily structures and tissue. Or else when you’ve gone through the airport and had to empty your pockets before going through security. However, while X-rays can pick up many things, until now, scientists haven’t been able to characterize a single atom using X-rays.
A team of scientists was led by Ohio University Professor of Physics and Argonne National Laboratory scientist, Saw Wai Hla.
In a project funded by the US Department of Energy, Office of Basic Energy Sciences, the team set about finding a way to get a singular atom picked up and able to be charactized using X-rays – the smallest amount to date being 10,000 atoms or more, as per a news release by Ohio University on Eurek Alert.
But why is picking up a singular atom on an X-ray so important?
Until now, the smallest number of atoms X-rays could pick up was 10,000 (Jonathan Kitchen/ Getty Stock Images)
Why
Well, Hla explains without X-rays there’s no way of telling what an atom is ‘made of’.
An X-ray of a single atom has been compared to being like the image of a fingerprint and way of identifying not just its physical but chemical properties too.
Until the project, atoms could be ‘routinely imaged with scanning probe microscopes’ but scientists couldn’t detect the exact ‘type of a particular atom, one atom-at-a-time’ alongside measuring its chemical state.
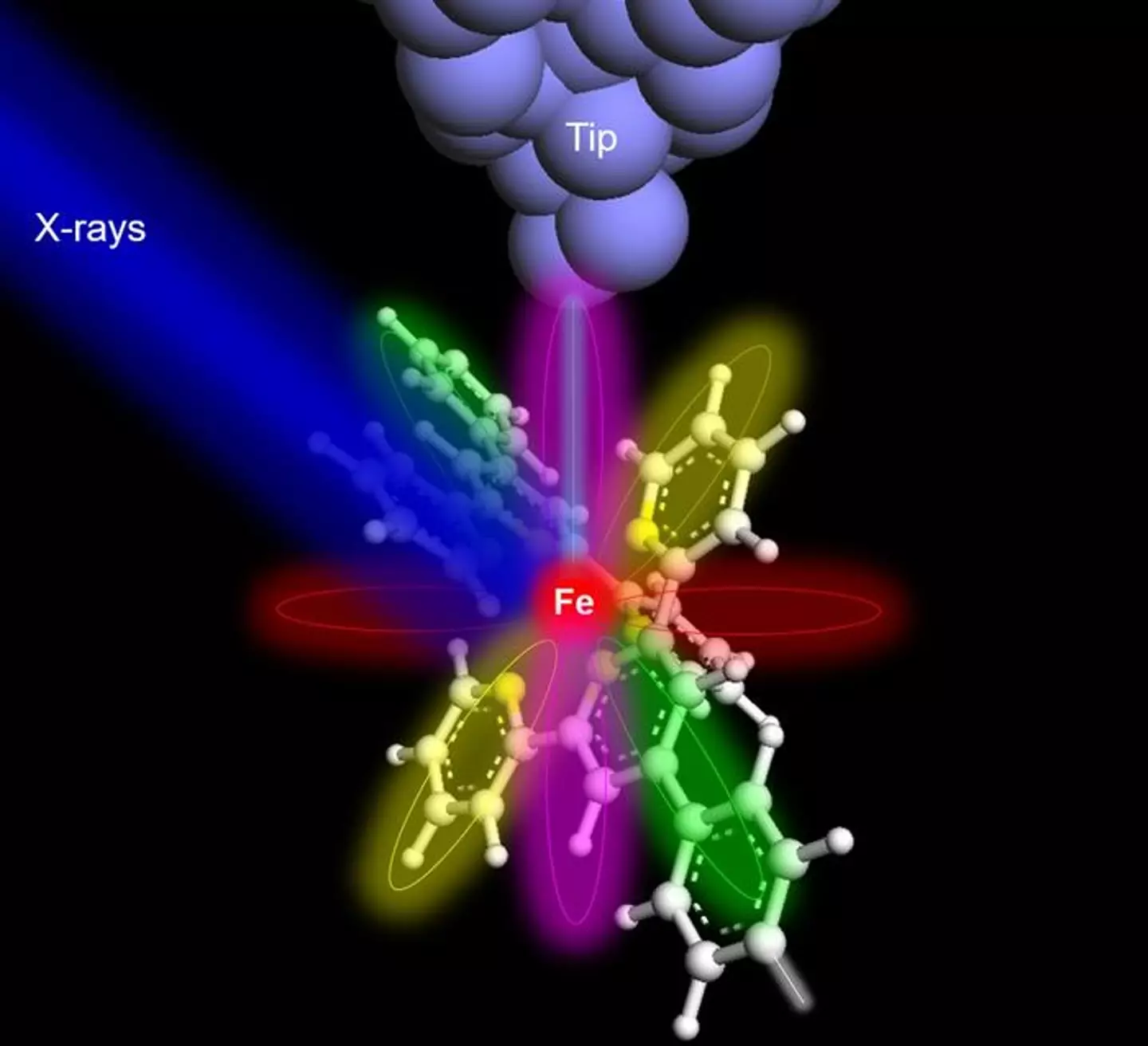
The technique the team used to successfully got an X-ray image of a singular atom (Saw-Wai Hla/ Ohio University)
How
With the help of a purpose-built synchrotron X-ray instrument, the scientists set about testing the device on an iron atom and a terbium atom in Argonne National Laboratory at the Center for Nanoscale Materials.
The team inserted each individual atom into ‘respective molecular hosts’. To detect the X-ray signal of one atom, the team supplemented ‘conventional detectors in X-rays with a specialized detectors made of a sharp metal tip positioned at extreme proximity to the sample to collect X-ray excited electrons’.
The technique is known as synchrotron X-ray scanning tunneling microscopy (SX-STM) and the team ‘broke new ground in X-ray science and nanoscale studies’ using it according to first author of the paper Tolilope Michael Ajayi.
And what’s more, the team achieved their goal – detecting a single atom X-ray signature, alongside detecting the chemical states of the individual atoms as well, finding that the terbium atom is ‘rather isolated and does not change its chemical state while the iron atom strongly interacts with its surroundings’.
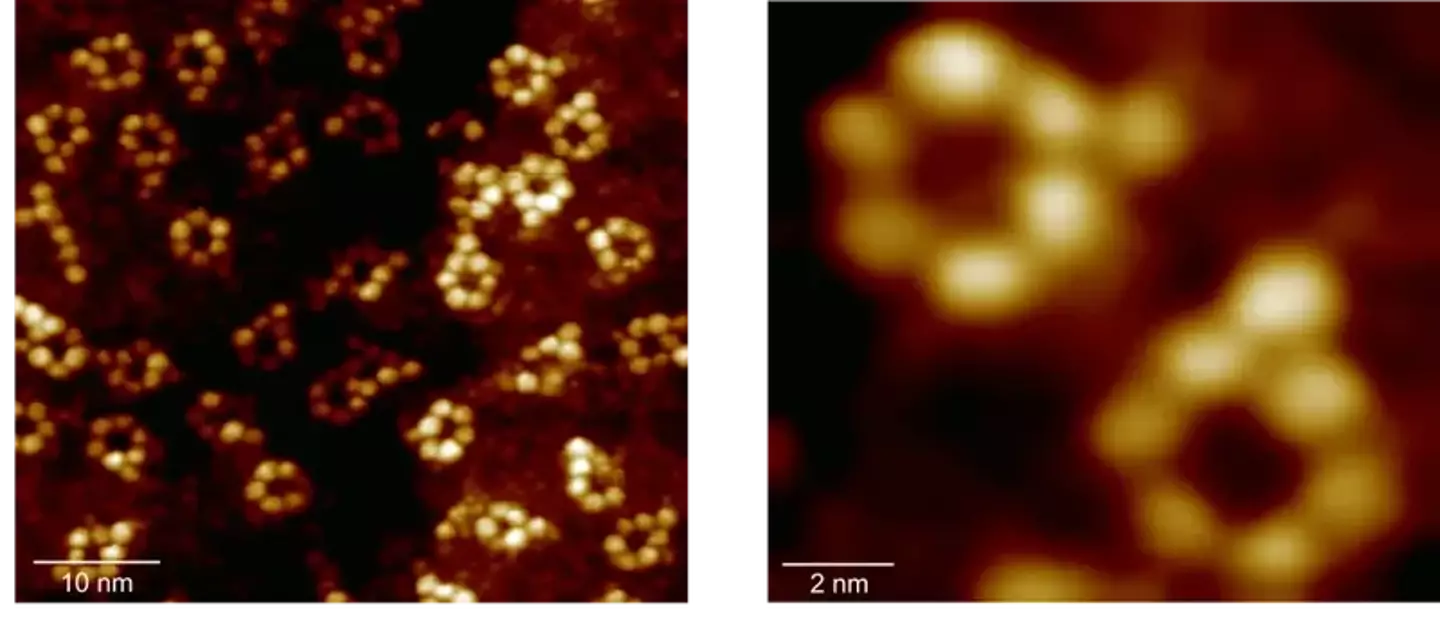
The X-ray technique revealed six rubidium atoms and an iron atom (Ajayi et al., Nature)
The study’s paper – titled Characterization of just one atom using synchrotron rays and. published in Nature – states: “Our work connects synchrotron X-rays with a quantum tunnelling process and opens future X-rays experiments for simultaneous characterizations of elemental and chemical properties of materials at the ultimate single-atom limit.
Hla resolved: “Once we are able to do that, we can trace the materials down to ultimate limit of just one atom. This will have a great impact on environmental and medical sciences and maybe even find a cure that can have a huge impact for humankind. This discovery will transform the world.”